With initial financial support from the National Manufacturing Institute (Scotland) through the National Transition Training Funding set up by the Scottish Government, the MDMC has put together a unique and innovative Continuing Professional Development (CPD) course in Medical Device Regulations and Healthcare Technology Device, Adoption & Management in Clinical Settings.
The course follows the BRUNEL™ toolkit created by Prof. Richard Scott, Honorary Professor at Heriot-Watt University and Director of Medical Physics at University Hospital Bristol & Weston NHS Trust Foundation. A picture of this model is given in Figure 1.
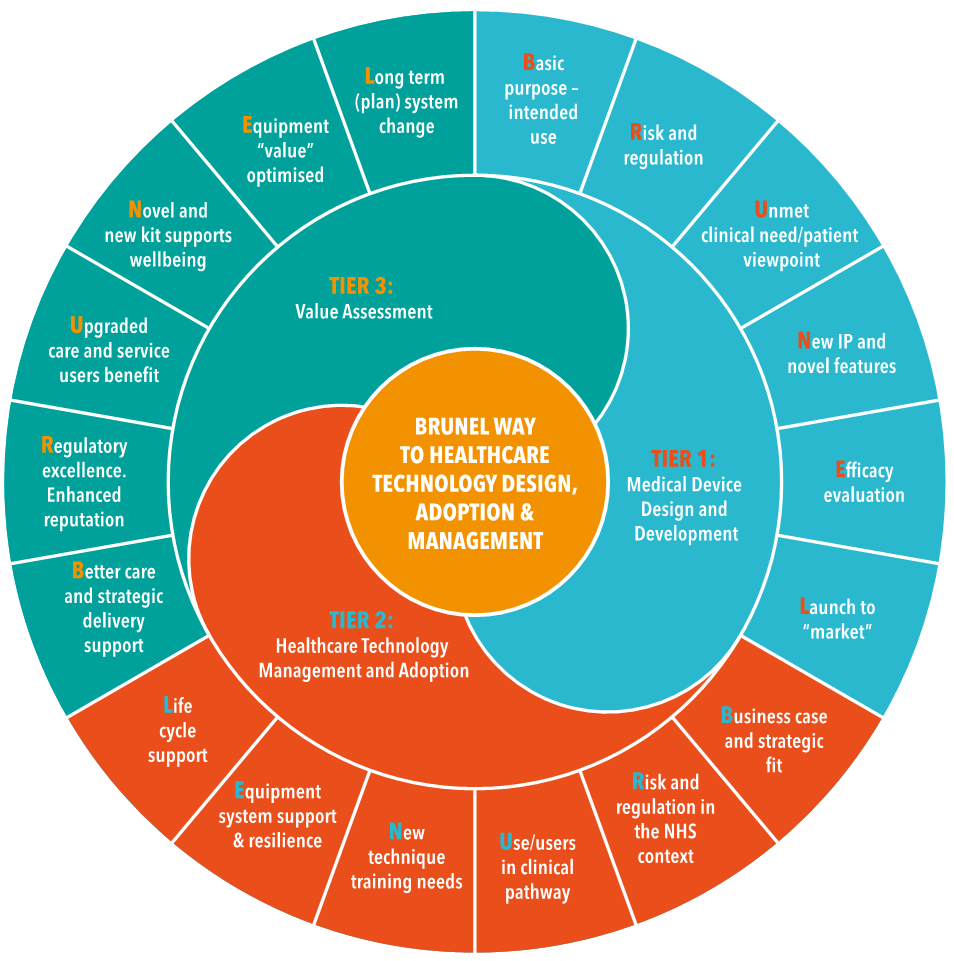
The course is free of charge to SME employees wishing to accelerate the adoption of their medical products into clinical settings.
Since April 2020, the CPD course has been delivered three times by experts from the MDMC, British Standards Institute, InnoScot Health, NIHR Device for Dignity MedTech Co-operative (Sheffield), and University Hospital Bristol & Weston NHS FT. Further deliveries are expected from September 2023.
